National Hemophilia Foundation (NHF) - Posters
The World Federation of Hemophilia World Bleeding Disorders Registry: A Two-year Update |
|
|
|
|
The World Federation of Hemophilia World Bleeding Disorders Registry: A Two-year Update
Objective:
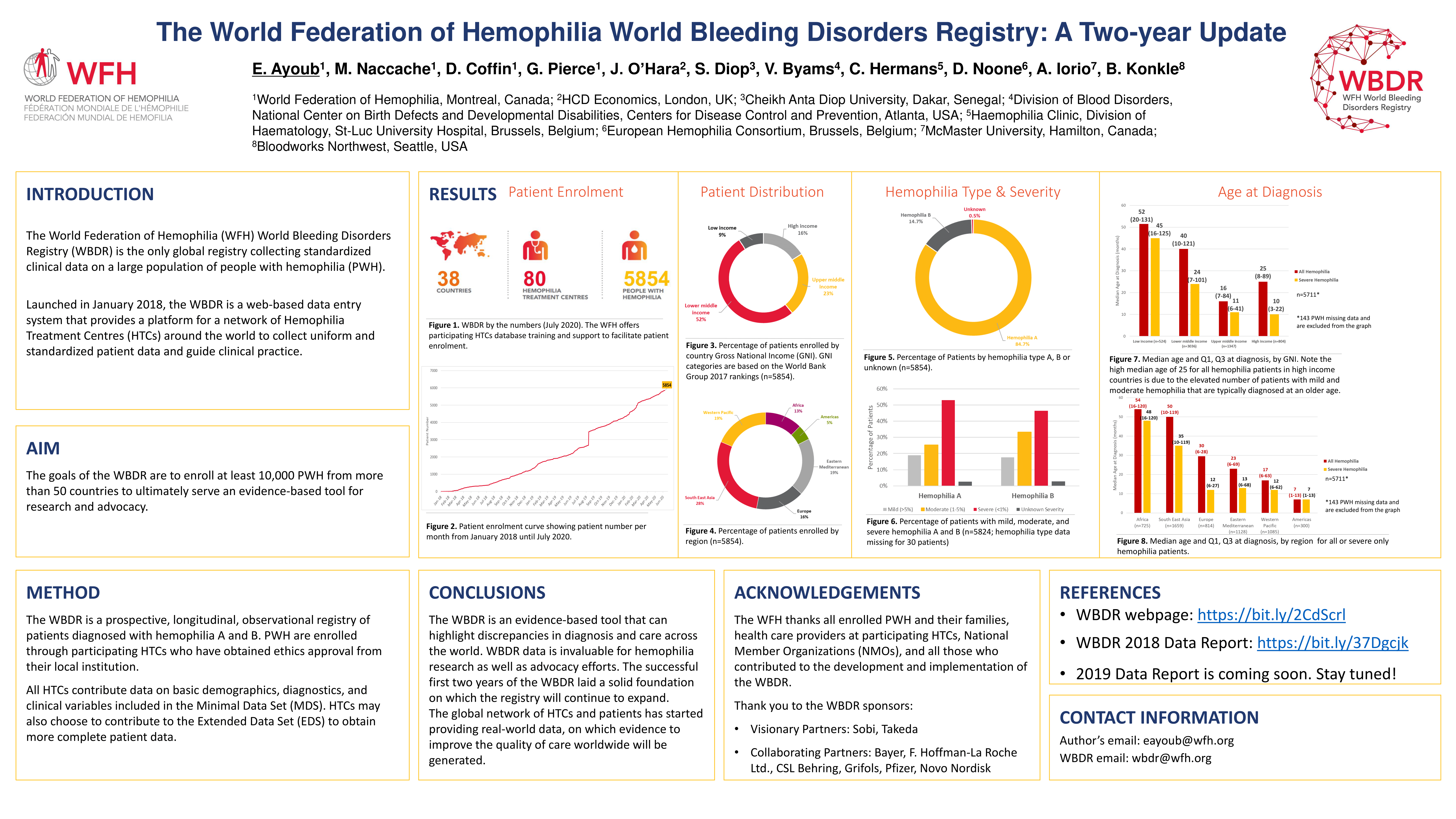
As a prospective, longitudinal, observational registry of patients with hemophilia (PWH) A and B, the World Bleeding Disorders Registry (WBDR) aims to collect uniform and standardized patient data and guide clinical practice across the world. The WBDR was established in January 2018 by the World Federation of Hemophilia (WFH), with a five-year target of 10,000 patients at 200 Hemophilia Treatment Centres (HTCs) in at least 50 countries.
Methods:
Data collection occurs at the level of participating HTCs and include data on basic demographics, diagnostics, and clinical variables included in the Minimal Data Set, in addition to an Extended Data Set to obtain more complete patient data. All data entered in the WBDR are quality assessed through the WFH Data Quality Accreditation Program. Additionally, an international data integration component was developed to conduct data transfer from existing patient registries to the WBDR.
Summary:
In the first two years, 80 HTCs from 38 countries have received ethics approval. Over 5200 PWH have been enrolled, including 771 patients imported directly from the Czech National Hemophilia Program Registry via data transfer. Patients registered in the WBDR represent all regions of the world and all World Bank Gross National Income (GNI) categories. Trends in global care around the world are beginning to emerge from this early data: the median age at diagnosis for severe patients, an indicator of the level of care in a country, varies considerably based on GNI: ranging from 45 months in low income countries, to 25, 12 and 10 in lower-middle, upper-middle and high income countries respectively. Bleed rates, treatment regimens and factor use also highlight large discrepancies in care globally.
Conclusions:
The significant progress the WBDR has accomplished in its first two years laid a solid foundation on which the registry will continue to expand. This global network of HTCs and patients has started providing real-world data, on which evidence to improve the quality of care worldwide will be generated.
The WFH thanks the many dedicated health care providers and patients who are part of this important initiative.
The WBDR is supported by our Visionary Partners: SOBI and Takeda; and our Collaborating Partners: Bayer, CSL Behring, Grifols, Pfizer, and Roche.



