National Hemophilia Foundation (NHF) - Posters
Development of the WFH-ISTH Gene Therapy Registry |
|
|
|
|
Objective:
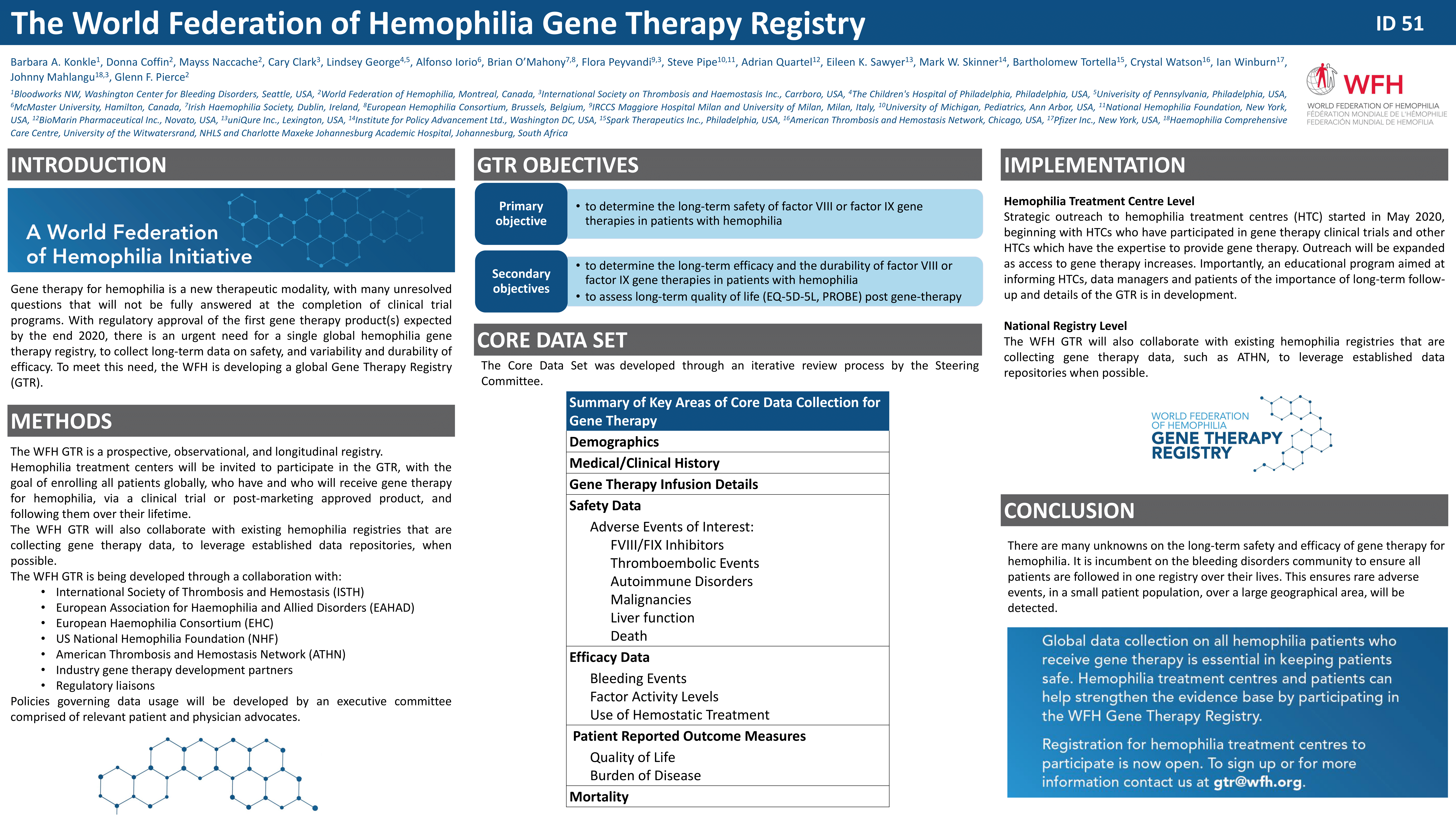
Gene therapy for hemophilia is a new therapeutic modality, with many unresolved questions that will not be fully answered at the completion of clinical trial programs. With regulatory approval of the first gene therapy product(s) expected by the end of 2020, there is an urgent need for a single global hemophilia gene therapy registry, to collect long-term data on safety, and variability and durability of efficacy.
Through a collaboration with the International Society of Thrombosis and Hemostasis (ISTH), the European Haemophilia Consortium (EHC), the US National Hemophilia Foundation (NHF), the American Thrombosis and Hemostasis Network (ATHN), industry gene therapy development partners and regulatory liaisons, the WFH is developing a global Gene Therapy Registry (GTR).
The aim of the WFH-ISTH GTR project is to provide a robust, scientifically valid data collection avenue, available to all health care providers treating patients who receive gene therapy. A core data set, developed by theGTR steering committee, will be used to collect standardized data.
Methods:
The WFH-ISTH GTR is a prospective, observational, and longitudinal registry. Hemophilia treatment centres (HTC) will be invited to participate in the GTR, with the goal of enrolling all patients globally, who have and who will receive gene therapy for hemophilia, via a clinical trial or post-marketing approved product, and following them over their lifetime. Strategic outreach to HTCs will start in mid-2020, beginning with HTCs who have participated in gene therapy clinical trials and other HTCs which have the expertise to provide gene therapy. Outreach will be expanded as access to gene therapy increases. The WFH-ISTH GTR will also collaborate with existing hemophiliaregistries that are collecting gene therapy data, to leverage established data repositories, when possible. Importantly, an educational program aimed at informing HTCs, data managers and patients of the importance of long-term follow-up and details of the GTR is in development. Components of the educational program include on-line training, user guides, best-practices, delivered via webinars, face-to-face meetings held in conjunction with larger conferences and in-person HTC visits, when possible.
The data stemming from the WFH-ISTH GTR will provide for robust ongoing surveillance of safety and efficacy. The database will be developed in Q3-Q4 2020, aiming for a start date in Q1 2021.
Conclusions:
There are many unknowns on the long-term safety and efficacy of gene therapy for hemophilia. It is incumbent on the bleeding disorders community to ensure all patients are followed in one registry over their lives. This ensures rare adverse events, in a small patient population, over a large geographical area, will be detected.



