National Hemophilia Foundation (NHF) - Posters
Vector DNA clearance from bodily fluids in patients with severe or moderate-severe hemophilia B following systemic administration of AAV5-hFIX and AAV5-hFIX Padua |
|
|
|
|
Objective:
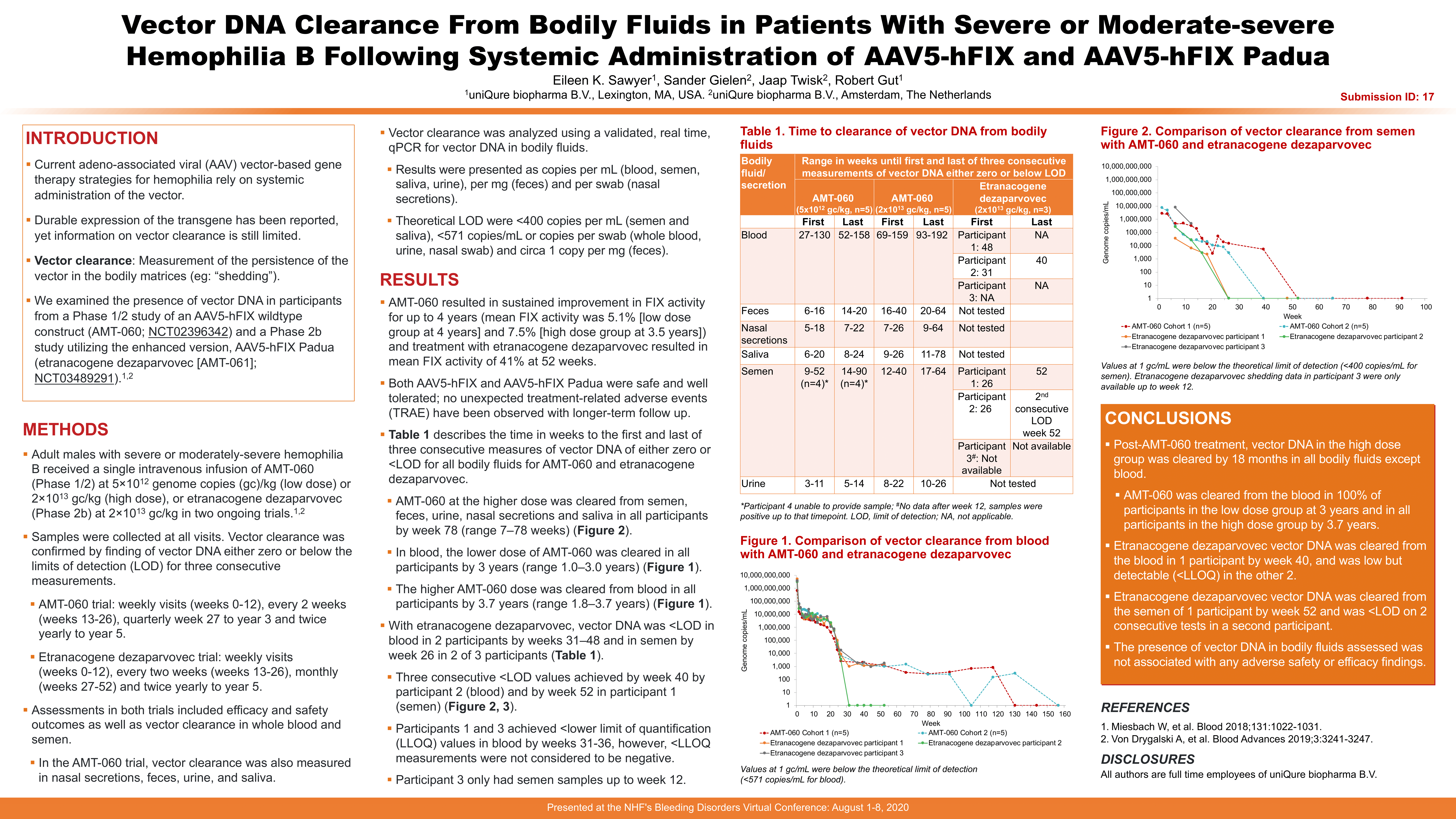
Adeno-associated viral (AAV) vector-based gene therapy strategies for hemophilia rely on systemic administration. Durable transgene expression has been reported from several trials, yet information on clearance of vector material from bodily fluids is limited. Monitoring of “shedding is required during trials despite lack of evidence of environmental or transmission risk. We aimed to examine the magnitude and duration of the presence of vector DNA in bodily fluids from participants in two ongoing trials: a Phase I/II study of an AAV5-hFIX wildtype construct (AMT-060; NCT02396342), and a Phase IIb study utilizing the enhanced version, AAV5-hFIX Padua (AMT-061; NCT03489291).
Methods:
Adult male participants with severe/moderately severe hemophilia B received a single intravenous infusion of either AMT-060 at 5x1012 genome copies(gc)/kg (low dose) or 21013 gc/kg (high dose), or AMT-061 at 2x1013gc/kg. Assessments included efficacy and safety outcomes, and vector shedding in whole blood and semen. In participants receiving AMT-060, vector shedding was also measured in nasal secretions, feces, urine, and saliva. Vector shedding was analyzed using a validated qPCR-based assay measuring vector DNA in the bodily fluids. Vector clearance was reached when vector DNA was zero or below the limit of detection (LOD) for three consecutive measurements. The range in time to the first and third consecutive negative measurement are provided.
Summary:
Treatment with AMT-060 resulted in sustained improvement in FIX activity for up to 4 years [mean FIX activity: 5.1% (low dose, 4 years); 7.5% (high dose, 3.5 years)]. Treatment with AMT-061 resulted in mean FIX activity of 41% at 52 weeks. AMT-060 and AMT-061 reduced the mean number of annualized bleeds by 77%-100% respectively and reduced the requirement for exogenous FIX administration by 90%-100% respectively. Both treatments were safe and well tolerated with no unexpected treatment-related adverse events observed during longer-term follow up. Higher dose AMT-060 was cleared from semen, feces, urine, nasal secretions and saliva in all participants by week 78 (range 7-78 weeks) and blood by 3.7 years (range 1.8-3.7 years). AMT-061 vector DNA was cleared from blood in 1 participant at 40 weeks and was low but detectable (<LLOQ) in the other 2 participants. AMT-061 vector DNA was cleared in the semen of 1 participant by week 52 and was <LOD on 2 consecutive tests in a second participant.
Conclusions:
After high dose AMT-060, vector DNA was undetectable in all participants by 10 months and considered cleared by 18 months in all bodily fluids except blood (which was cleared in all participants by 3.7 years). With AMT-061, vector DNA was cleared from blood in 1 participant by week 40 and from semen in 1 participant by week 52. The presence of vector DNA in bodily fluids was not associated with any adverse safety or efficacy findings.



